MARCH 2023
SCIENCE AND TECHNOLOGY PAPER-I
(REVISED COURSE)
Q. 1. (A) Choose the correct alternative :
(i) The device used for producing current is called……………………….
(A) A voltmeter
(B) An ammeter
(C) A galvanometer
(D) A generator
(ii) If a ray of light passes from a denser medium to a rarer medium in a straight line, the angle of incidence must be………………..
(A)
(B)
(C)
(D)
(iii) The power of convex lens of focal length is………………………..
(A)
(B)
(C)
(D)
(iv) Good conductor of electricity is……………………………
(A) Bromine
(B) Iodine
(C) Graphite
(D) Sulphur
(v) The height of medium earth orbit above surface of the earth is :
(A)
(B)
(C)
(D)
(B) Answer the following questions :
(i) Find the odd man out :
Loudspeaker, Microphone, Electric motor, Magnet.
(ii) Complete the correlation :
: Brown : : :…………………..
(iii) Match the pair :
Group ‘A’ Group ‘B’
Substance Refractive index
Air (a) 1.33
(b) 1.46
(c) 1.0003
(iv) State True or False :
“Wavelength of red light is close to
(v) Write the name of small satellite made by a group of students from COEP (College of Engineering, Pune) sent to the space through ISRO in 2016.
Answer:
1.(A) (i) (D) The device used for producing current is called A generator.
(ii) (A) If a ray of light passes from a denser medium to a rarer medium in a straight line, the angle of incidence must be .
(iii) (A) The power of convex lens of focal length is .
(iv) (C) Good conductor of electricity is Graphite.
(v) (D) The height of medium earth orbit above surface of the earth is .
(B)
(i) Magnet
(ii) Brown : White.
(iii) Match the pair.
Group ‘A’ Group ‘B’
Substance Refractive index
Air (c) 1.0003
(iv) “Wavelength of red light is close to ” True
(v) Swayam Satellite
Q. 2. (A) Give scientific reasons (any two) :
(i) For electric power transmission, copper or aluminium wire is used.
(ii) Lemon or tamarind is used for cleaning copper vessels turned greenish.
(iii) Elements belonging to the same group have the same valency.
(B) Answer the following questions (any three) :
(i) How do we feel about air in each of the following conditions?
(a) Relative humidity is more than .
(b) Relative humidity is less than .
(ii) Complete the following reaction :
+ .
(iii) Distinguish between Mass and Weight.
(iv) Complete the following table :
Type of Satellite |
| ||||
|
| ||||
|
|
(v) Define periods and groups of modern periodic table.
Answer:
2. (A)
(i)Copper and aluminium contain a large number of free electrons. These free electrons can move throughout conductor easily. This results into copper and aluminium having low values of resistivity.
Thus, copper and aluminium are good conductors of electricity and offer low resistance to the flow of current. Hence, copper or aluminium wire is used for electric power transmission.
(ii) Copper vessels turned greenish due to the formation of copper carbonate layer. The citric acid present in the lemon or tamarind neutralises the basic copper carbonate and dissolves the layer. That is why tarnished copper vessels are cleaned with lemon or tamarind juice, to give the surface of copper vessel its characteristic lustre.
(iii) The valency of an element is determined by the number of valence electrons in the outermost shell of an atom of an element. All the elements in a group have the same number of valence electrons. Therefore, elements in the same group have the some valency.
(B)
(i) (a) If the relative humidity is more than , we feel that the air is humid.
(b) If the relative humidity is less than , we feel that the air is dry.
(ii)
(iii) | Mass | Weight | ||||||||
1. |
| 1. |
| |||||||
2. |
| 2. |
| |||||||
3. | Mass is the measure of inertia. | 3. | Weight is the measure of force. | |||||||
4. |
| 4. | The SI unit of weight is the Newton (N). | |||||||
5. |
| 5. |
|
(iv) | Types of Satellite | The names of Indian Satellite and launcher | |||||
1. | Navigational Satellite |
| |||||
2. | Earth Observation Satellite |
|
(v) Periods : The horizontal rows are called periods. There are seven periods in the periodic table.
Groups : Vertical Columns in the periodic table starting from top to bottom are called groups. There are 18 groups in modern periodic table.
Q. 3. Answer the following question (any five) :
(i) Calculate the escape velocity on the surface of the moon given the mass and radius of the moon to be and respectively. (Given : ).
(ii) An element has its electron configuration as 2,8,1. Now answer the following questions :
(a) What is the atomic number of this element?
(b) What is the group of this element ?
(c) To which period does the element belong?
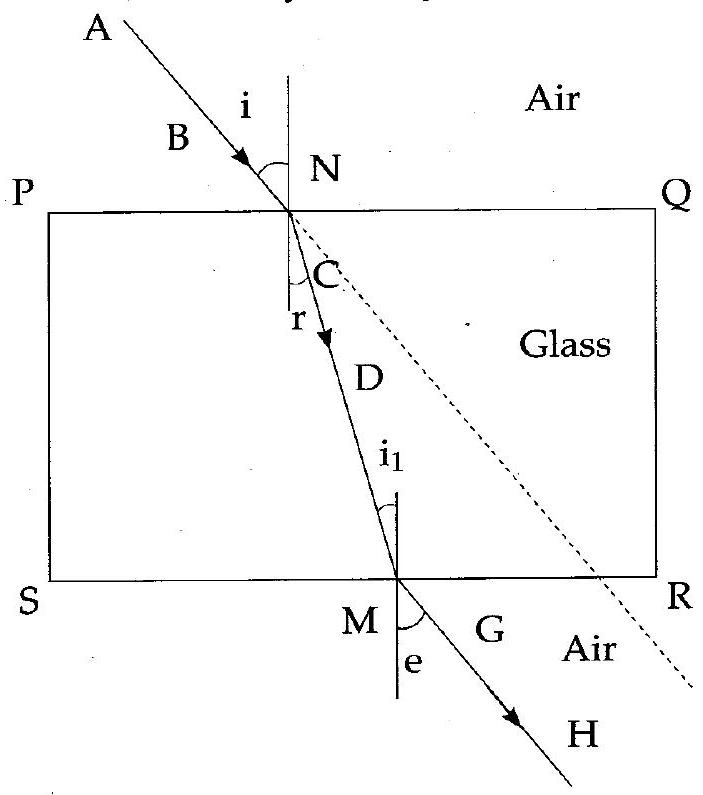
(iii) Observe the figure and name the ray , ray , ray :
(iv) Read the following sentence and answer the questions :
” is an ionic compound?
(a) Why is an ionic compound?
(b) State any two properties of ionic compounds.
(v) Identify the physical and chemical changes from the following phenomena :
(a) Transformation of ice into water.
(b) Ripening of fruit.
(c) Milk turned into curd.
(d) Evaporation of water.
(e) Digestion of food in the stomach.
(f) Iron fillings get attracted towards the magnet.
(vi) Observe the following figure and answer the questions :
Wax layer
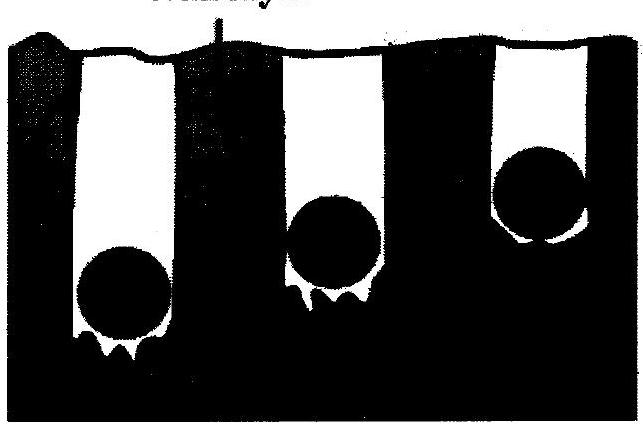
Specific heat capacity of metals
(a) Which element has maximum specific heat capacity? Justify.
(b) Which element has minimum specific heat capacity? Justify.
(c) Define specific heat of object.
(vii) Identify figures and give their uses :
(a)
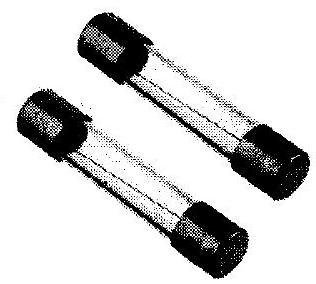
(b)
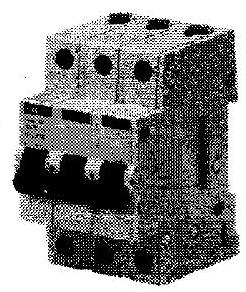
(c)
(viii) Complete the following flow chart :
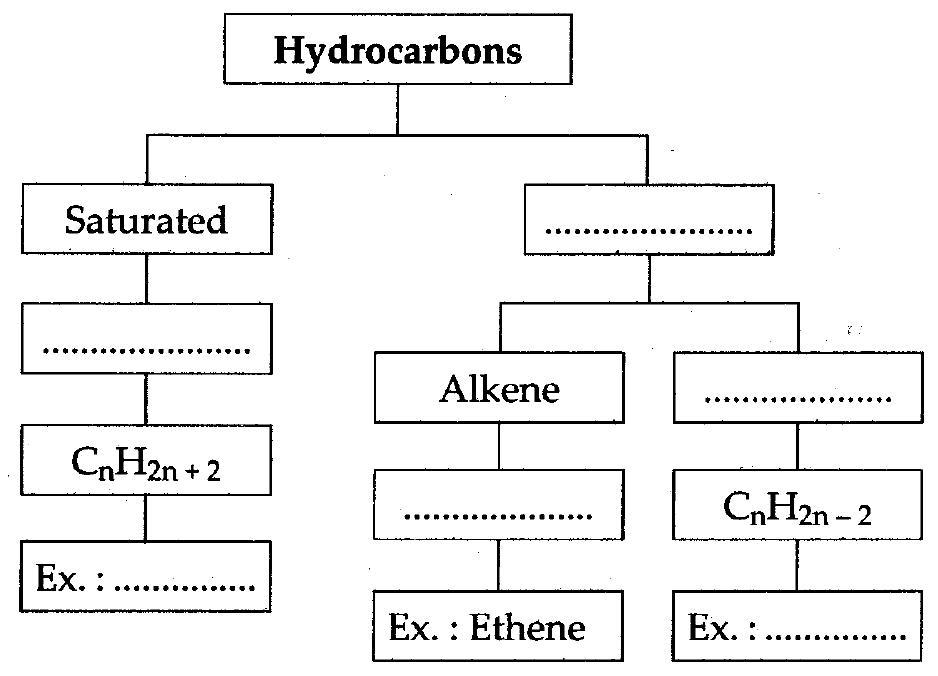
Answer:
3.
(i) Given :
Mass of moon,
Radius of the moon,
We know,
Escape velocity,
Thus, The escape velocity on the surface of the moon is .
(ii) An element has its electronic configuration as 2, 8, 1 .
(a) The atomic number of this element i.e. sodium is 11.
(b) This element is sodium and it belongs to Group I of the periodic table. This group consists of Alkali metals.
(c) The period to which sodium belongs is Period 3.
(iii) Ray AB – Incident Ray
Ray CD – Refracted Ray
Ray GH – Emergent Ray
(iv) (a) – The electronic configuration of sodium is 2, 8, 1 and the electronic configuration of chlorine is . For stability sodium atom donates it’s one electron to chlorine and the chlorine atom gains it, which causes the formation of the sodium chloride compounds. Since sodium chloride is formed by the transfer of electrons between the elements, therefore it is an ionic compound.
(b) Two properties of ionic compounds :
- Ionic compounds have high melting as well as boiling points.
- They are hard and brittle in nature.
(v) (a) Transformation of ice into water – Physical change
(b) Ripening of fruit- Chemical change
(c) Milk turned into card – Chemical change
(d) Evaporation of water – Physical change
(e) Digestion of food in the stomach – Chemical change
(f) Iron Fillings get attracted towards the magnet – Physical change
(vi) (a) Iron has maximum specific heat capacity. In the given activity heat absorbed by iron sphere is transmitted more in the wax, hence sphere goes deepest into wax, while lead sphere absorbs less heat, resulting in less transmission of heat in the wax hence sphere goes the least depth in the wax.
(b) Lead has minimum specific heat capacity. Lead sphere absorbs less heat, resulting in less transmission of heat in the wax hence sphere goes the least depth in the wax.
(c) Specific heat of object : The amount of heat that is required to increase the temperature of unit mass of that particular substance through one degree is called specific heat of object.
(vii) (a) Figure : Cartridge type fuse/Electric fuse.
Uses – 1. It is a safety device that protects the wiring against excessive heating caused by an excess supply of current.
- It melts when heavy current flows though the circuit, thereby causing the circuit to be broken and current ceases to flow through it.
(b) Figure : MCB (Miniature Circuit Breaker)
Uses – An MCB is a device which functions as a fuse but does not require replacement. MCB falls down to break the circuit when heavy amount of current flows through it. Once the fault is rectified, MCB is reset.
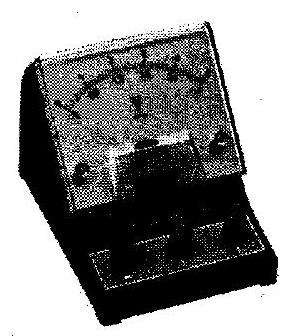
(c) Figure-Galvanometer
Uses – It is used to measure the voltage by connecting it in series with high resistance.
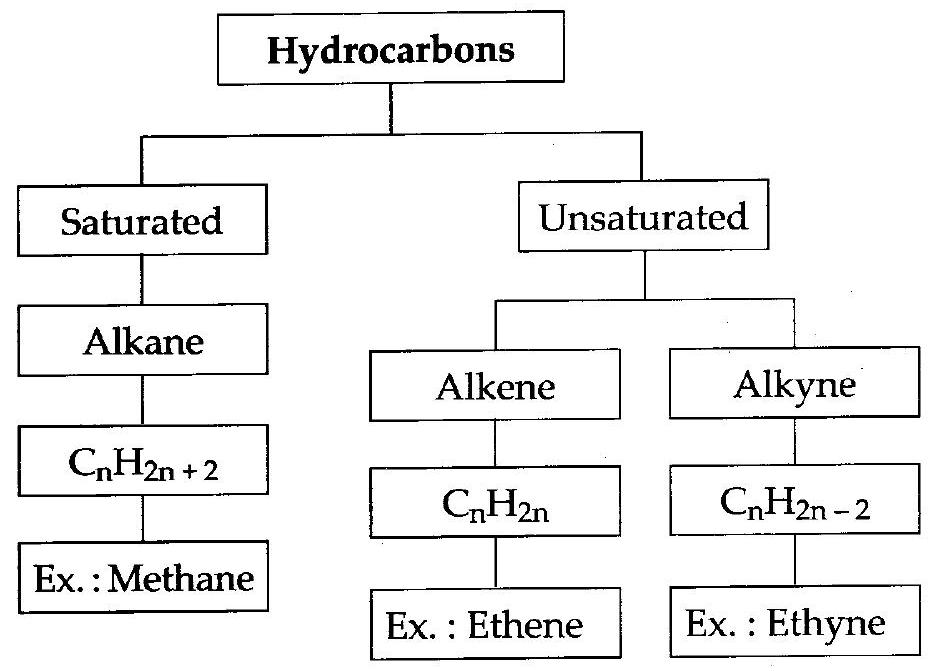
(viii)
Q. 4. Answer any one of the following questions:
(i) Observe the following diagram and answer the questions given below :

(a) Name the defect of vision represented in above figure
(b) State the reasons for this defect.
(c) How is it corrected?
(d) Draw the diagram to show the correction of this defect.
(ii) Complete the following table
S.N. | Common Name | Structural Formula | IUPAC Name |
1. | Ethylene | ||
2. | Acetylene | Ethyne | |
3. | Acetic acid | ||
4. | Methyl alcohol | Propane-2-one | |
5. |
Answer:
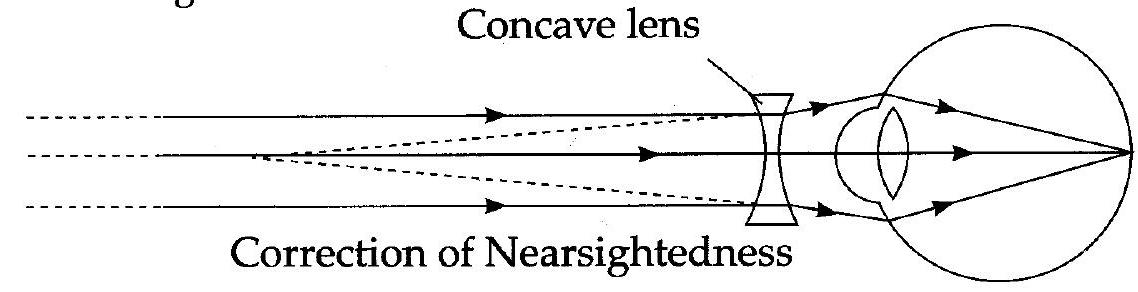
- (i) (a) In the figure, Myopia or Nearsightedness defect of vision is represented.
(b) The reasons for this defect:
- The curvature of the cornea and the eyelens increases. The muscles near the lens cannot relax so that the converging power of the lens remains large.
- The eyeball elongates so that the distance between the lens and the retina increases.
(c) This defect can be corrected using spectacles with concave lens. This lens diverges the incident rays and these diverged rays can be converged by the lens in the eye to form image on the retina.
(d)
(ii) A
S. No | Common Name | Structural Formula | IUPAC Name |
1. | Ethylene | Ethene | |
2. | Acetylene | Ethyne | |
3. | Acetic Acid | Ethanoic acid | |
4. | Methyl alcohol | Methanol | |
5. | Acetone | Propane-2-one |