Chapter 16 Chemistry in Everyday Life
1. Choose the correct option
Question A.
Oxidative Rancidity is …………….. reaction
a. addition
b. substitution
c. Free radical
d. combination
Answer:
c. Free radical
Question B.
Saponification is carried out by ……………..
a. oxidation
b. alkaline hydrolysis
c. polymerisation
d. Free radical formation
Answer:
b. alkaline hydrolysis
Question C.
Aspirin is chemically named as ……………..
a. Salicylic acid
b. acetyl salicylic acid
c. chloroxylenol
d. thymol
Answer:
b. acetyl salicylic acid
Question D.
Find odd one out from the following
a. dettol
b. chloroxylenol
c. paracetamol
d. trichlorophenol
Answer:
c. paracetamol
Question E.
Arsenic based antibiotic is
a. Azodye
b. prontosil
c. salvarsan
d. sulphapyridine
Answer:
c. salvarsan
Question F.
The chemical used to slow down the browning action of cut fruit is
a. SO3
b. SO2
c. H2SO4
d. Na2CO3
Answer:
b. SO2
Question G.
The chemical is responsible for the rancid flavour of fats is
a. Butyric acid
b. Glycerol
c. Protein
d. Saturated fat
Answer:
a. Butyric acid
Question H.
Health benefits are obtained by consumption of
a. Saturated fats
b. trans fats
c. monounsaturated fats
d. all of these
Answer:
c. monounsaturated fats
2. Explain the following :
Question A.
Cooking makes food easy to digest.
Answer:
- During the cooking process, high polymers of carbohydrates or proteins are hydrolysed to smaller polymeric units.
- The uncooked food mixture is a heterogeneous suspension which becomes a colloidal matter on cooking.
- As a result, the constituent nutrient molecules present in cooked food are smaller in size and hence, easier to digest, than the uncooked food.
Hence, cooking makes food easy to digest.
Question B.
On cutting some fruits and vegetables turn brown.
Answer:
i. Cutting of fruits and vegetables damage the cells, resulting in release of chemicals.
ii. Depending on the pH of fruits/vegetables, polyphenols are released.
iii. Due to the action of an enzyme, these polyphenols react with oxygen present in the air and get oxidised to form quinones.

iv. Quinones further undergo reactions including polymerization, which results in the formation of brown coloured products called as tannins.
Thus, on cutting, some fruits and vegetables turn brown.
Question C.
Vitmin E is added to packed edible oil.
Answer:
- Vitamin E is a very effective natural antioxidant.
- The phenolic – OH group present in the structure of vitamin E is responsible for its antioxidant activity.
- Also, the long chain of saturated carbon atoms makes it fat soluble.
Therefore, when vitamin E is added to packed edible oil, it prevents the oxidative rancidity of the oil.
Question D.
Browning of cut apple can be prolonged by applying lemon juice.
Answer:
- Browning of cut apple is due to the oxidation of polyphenols at a particular pH to quinones, which further undergoes polymerization to form brown coloured tannins.
- This browning reaction can be prolonged or slowed down by using reducing agents or by changing the pH.
- Applying lemon juice (i.e., citric acid) on the cut apple, lowers the pH at the surface of the apple. This prevents the oxidation reaction. Thus, browning of cut apple can be prolonged by applying lemon juice.
Question E.
A diluted solution (4.8 % w/v) of 2,4,6-trichlorophenol is employed as antiseptic.
Answer:
- 2,4,6-Trichlorophenol (TCP) is more potent antiseptic than phenol.
- It has low corrosive effects as compared to phenol, if used in lower concentrations.
Hence, diluted solution (4.8% w/v) of 2,4,6-trichlorophenol is used as antiseptic.
Question F.
Turmeric powder can be used as antiseptic.
Answer:
- Turmeric powder contains an active ingredient called curcumin.
- Curcumin has antiseptic properties; thus, it is used for wound healing or applied on bruise.
Hence, turmeric powder can be used as antiseptic.
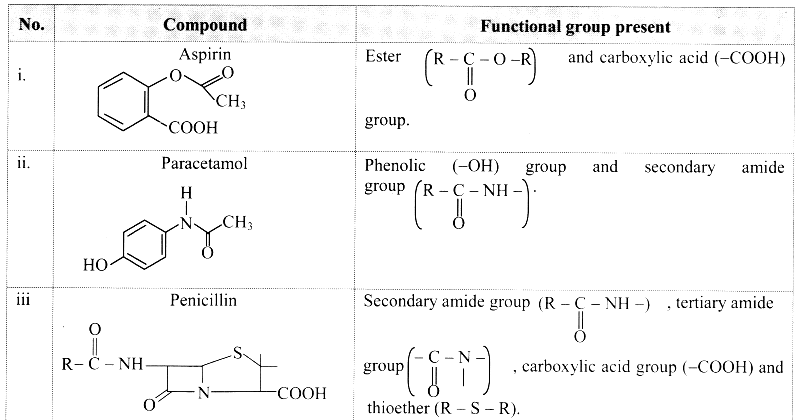
3. Identify the functional groups in the following molecule :
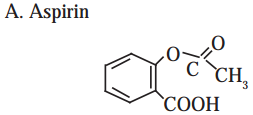

Answer:

4. Give two differences between the following
Question A.
Disinfectant and antiseptic
Answer:
Disinfectant | Antiseptic |
1. Disinfectants are applied on non-living surfaces like floors, instruments, sanitary ware, etc. to kill wide range of microorganisms. | 1. Antiseptics are applied on the surface of living tissues in order to sterilise them. |
2. Disinfectants cannot be applied on wounds. | 2. Antiseptics can be directly applied on wounds. |
3. p-chloro-o-benzyl phenol | 3. Iodine, boric acid, iodoform, dettol, etc. |
Question B.
Soap and synthetic detergent
Answer:
Soap | Synthetic detergent |
1. Soaps can be broadly classified into two types, i.e., toilet soaps (prepared using KOH) and laundry soaps (prepared using NaOH). | 1. Synthetic detergents are of three types, i.e., anionic, cationic and nonionic detergents. |
2. Soaps cannot be used in hard water. | 2. Synthetic detergents can be used in soft water as well as in hard water. |
Question C.
Saturated and unsaturated fats
Answer:
Saturated fats | Unsaturated fats |
1. In saturated fat, long chains of tetrahedral carbon atoms in the fatty acid get closely packed together. | 1. In unsaturated fats, the presence of one or more C = C bond in long chains of fatty acids, prevent molecules from packing closely together. |
2. In saturated fats, the van der Waals forces between long saturated chains are strong. Hence, their melting points are higher than unsaturated fats. | 2. In unsaturated fats, the van der Waals forces between long unsaturated chains are weak. Hence, their melting points are lower than saturated fats. |
Question D.
Rice flour and cooked rice
Answer:
Rice flour | Cooked rice |
1. Rice flour can be stored for a long period of time. It has a long shelf life. | 1. Cooked rice cannot be stored for a longer period of time. It has very short shelf life. |
2. Rice flour is uncooked food and hence, it is difficult to digest. | 2. Cooked rice is easier to digest. |
5. Match the pairs.
A group | B group |
A. Paracetamol | a. Antibiotic |
B. Chloramphenicol | b. Synthetic detergent |
C. BHT | c. Soap |
D. Sodium stearate | d. Antioxidant |
e. Analgesic |
Answer:
A – e,
B – a,
C – d,
D – c
6. Name two drugs which reduce body pain.
Answer:
Aspirin and paracetamol are the two drugs that reduce body pain.
7. Explain with examples
Question A.
Antiseptics
Answer:
i. Antiseptics are used to sterilise surfaces of living tissue when the risk of infection is very high, such as during surgery or on wounds.
ii. Commonly used antiseptics include inorganics like iodine and boric acid or organics like iodoform and some phenolic compounds.
e.g.
- Tincture of iodine (2-3% solution of iodine in alcohol-water mixture) and iodoform serve as powerful antiseptics and is used to apply on wounds.
- A dilute aqueous solution of boric acid is a weak antiseptic used for eyes.
- Various phenols are used as antiseptics. A dilute aqueous solution of phenol (carbolic acid) is used as antiseptic; however, phenol is found to be corrosive in nature. Many chloro derivatives of phenols are more potent antiseptics than the phenol itself. They can be used with much lower concentrations, which reduce their corrosive effects.
- Two of the most common phenol derivatives in use are trichlorophenol (TCP) and chloroxylenol (which is an active ingredient of antiseptic dettol).
- Thymol obtained from oil of thyme (a spice plant) has excellent non-toxic antiseptic properties.
Question B.
Disinfectant
Answer:
- Disinfectants are non-selective antimicrobials.
- They kill a wide range of microorganisms including bacteria.
- They are used on non-living surfaces for example, floors, instruments, sanitary ware, etc.
-
Various phenols can be used as disinfectants.
e.g. p-Chloro-o-benzyl phenol is used as a disinfectant in all-purpose cleaners.
Question C.
Cationic detergents
Answer:
Cationic detergents: These are quaternary ammonium salts having one long chain alkyl group.
e.g. Ethyltrimethylammonium bromide: [CH3(CH2)15 – N+(CH3)3]Br–
Question D.
Anionic detergents
Answer:
Anionic detergents: These are sodium salts of long chain alkyl sulphonic acids or long chain alkyl substituted benzene sulphonic acids.
e.g. Sodium lauryl sulphate: CH3(CH2)10CH3OSO−3Na+
Question E.
Non-ionic detergents
Answer:
Nonionic detergents: These are ethers of polyethylene glycol with alkyl phenol or esters of polyethylene glycol with long chain fatty acid.
e.g. a. Nonionic detergent containing ether linkage:
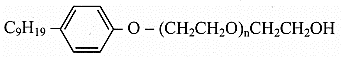
b. Nonionic detergent containing ester linkage: CH3(CH2)16 – COO(CH2CH2O)nCH2CH2OH
8. Explain : mechnism of cleansing Action of soap with flow chart.
Answer:
The following flow chart shows mechanism of cleansing action of soap:

9. What is meant by broad spectrum antibiotic and narrow spectrum antibiotics?
Answer:
Antibiotics which are effective against wide range of bacteria are known as broad spectrum antibiotics, while antibiotics which are effective against one group of bacteria are known as narrow spectrum antibiotics.
10. Answer in one senetence
Question A.
Name the painkiller obtained from acetylation of salicyclic acid.
Answer:
Aspirin is the pain killer obtained from acetylation of salicylic acid.
Question B.
Name the class of drug often called as painkiller.
Answer:
Analgesics are the class of drug often called as painkiller.
Question C.
Who discovered penicillin?
Answer:
Alexander Fleming discovered penicillin.
Question D.
Draw the structure of chloroxylenol and salvarsan.
Answer:
Structure of chloroxylenol:
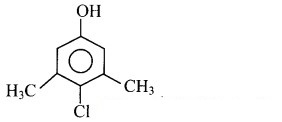
Structure of salvarsan:
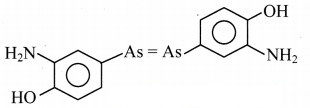
Question E.
Write molecular formula of Butylated hydroxy toulene.
Answer:
Molecular formula of butylated hydroxytoluene is C15H24O.
Question F.
What is the tincture of iodine ?
Answer:
Tincture of iodine is a 2-3% solution of iodine in alcohol-water mixture.
Question G.
Draw the structure of BHT.
Answer:

Question I.
Write a chemical equation for saponification.
Answer:
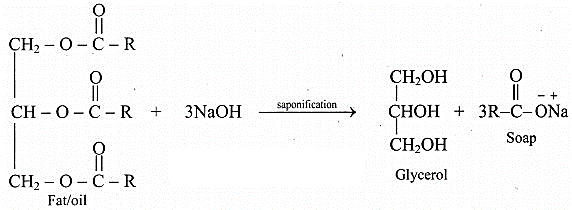
Question J.
Write the molecular formula and name of
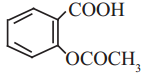
Answer:
Molecular formula: C9H8O4
Name: Aspirin
11. Answer the following
Question A.
Write two examples of the following.
a. Analgesics
c. Antiseptics
d. Antibiotics
e. Disinfectant
Answer:
No. | Drug type | Examples |
i. | Analgesics | Aspirin, paracetamol |
ii. | Antiseptics | Dettol, thymol |
iii. | Antibiotics | Penicillin, sulphapyridine |
iv. | Disinfectant | Phenol, p-Chloro-o-benzyl phenol |
Question B.
What do you understand by antioxidant ?
Answer:
- An antioxidant is a substance that delays the onset of oxidant or slows down the rate of oxidation of foodstuff.
- It is used to extend the shelf life of food.
-
Antioxidants react with oxygen-containing free radicals and thereby prevent oxidative rancidity.
e.g. Vitamin E is a very effective natural antioxidant.
Activity :
Collect information about different chemical compounds as per their applications in day-to-day life.
Answer:
No. | Chemical compound | Applications |
i. | Vinegar(CH3COOH) | Preservation of food, salad dressing, sauces, etc. |
ii. | Magnesium hydroxide [Mg(OH)2] | Common component of antacids (used to relieve heartburn, acid indigestion and stomach upset.) |
iii. | Baking soda (NaHCO3) | Cooking, antacid, toothpaste, etc. |
iv. | Sodium benzoate (C6H5COONa) | Used as food preservative |
[Note: Students can use the above information as reference and collect additional information on their own.]
Intext Questions and Answers
Can you recall? (Textbook Page No. 261)
Question i.
What are the components of balanced diet?
Answer:
Carbohydrates, proteins, lipids (fats and oil), vitamins, minerals and water are the components of balanced diet.
Question ii.
Why is food cooked? What is the difference in the physical states of uncooked and cooked food?
Answer:
- Food is cooked in order to make it easy to digest.
- Also, the raw or uncooked food may contain harmful microorganisms which may cause illness. Cooking of food at high temperature kills most of these microorganisms.
- Raw/uncooked food materials like dried pulses, vegetables, meat, etc. are hard and thus, not easily chewable while cooked food is soft and tender, therefore, easily chewable.
Question iii.
What are the chemicals that we come across in everyday life?
Answer:
Detergents, shampoos, medicines, various food flavours, food colours, etc. are different types of chemicals that we come across in everyday life.
Just think (Textbook Page No. 261)
Question i.
Why is food stored for a long time?
Answer:
Food (like various cereals, pulses, pickles) is stored for a long time to make it available in all seasons.
Question ii.
What methods are used for preservation of food?
Answer:
Various physical and chemical methods are used for preservations of food.
- Physical methods like, addition of heat, removal of heat, removal of water, irradiation, etc., are used in order to preserve food.
- Chemical methods like, addition of sugar, salt, vinegar, etc. are employed for preservation of food.
Question iii.
What is meant by quality of food?
Answer:
Food quality can be described in terms of parameters such as flavour, smell, texture, colour and microbial spoilage.
Can you recall? (Textbook Page No. 263)
Question i.
How is Vanaspati ghee made?
Answer:
Vanaspati ghee is prepared by hydrogenation of oils. Hydrogen gas is passed through the oils at about 450 K in the presence of nickel catalyst to form solid edible fats like vanaspati ghee.
![]()
Question ii.
What are the physical states of peanut oil, butter, animal fat, Vanaspati ghee at room temperature?
Answer:
Example | Physical state |
Peanut oil | Liquid |
Butter | Semi-solid |
Animal fat | Solid/semi-solid |
Vanaspati ghee | Solid/semi-solid |
Can you tell? (Textbook Page No. 264)
Question 1.
When is an antipyretic drug used?
Answer:
An antipyretic drug is used to reduce fever (that is, it lowers body temperature when a fever is present).
Question 2.
What type of medicine is applied to a bruise?
Answer:
Antiseptic such as tincture of iodine is applied on a bruise in order to prevent the exposed living tissue from getting infected.
Question 3.
What is meant by a broad spectrum antibiotic?
Answer:
Antibiotics which are effective against wide range of bacteria are known as broad spectrum antibiotic.
Question 4.
What is the active principle ingredient of cinnamon bark?
Answer:
Cinnamaldehyde is the principle active ingredient of cinnamon bark.
Can you tell? (Textbook Page No. 268)
Question i.
Can we use the same soap for bathing as well as cleaning utensils or washing clothes? Why?
Answer:
No, we cannot use the same soap for bathing as well as cleaning utensils or washing clothes due to the following reasons:
- Chemical composition of each type of soap or cleansing material is different.
- Nature, acidity, texture, reactivity towards water (i.e., hard water or soft water), reactivity towards microorganisms, stains are different for each type of soap.
-
Depending on these qualities, soaps are classified and used accordingly.
e.g. pH of soaps used for bathing purpose is different than that of the soap which is used for cleaning utensils.
Thus, we cannot use the same soap for bathing as well as cleaning utensils or washing clothes.
Question ii.
How will you differentiate between soaps and synthetic detergent using borewell water?
Answer:
Borewell water is hard water. Soaps and synthetic detergents react differently with hard water.
- Soap: Soaps are insoluble in hard water. Borewell water (hard water) contains Ca2+ and Mg2+ ions. Soaps react with these ions to form insoluble magnesium and calcium salts of fatty acids. These salts precipitate out as gummy substance or form scum.
- Synthetic detergents: Synthetic detergents can be used in hard water as well. They contain molecules (components) which form soluble calcium and magnesium salts.
Thus, soaps will form scum in borewell water but synthetic detergents will not.