Chp 1 Solutions board exam questions
HSC Exam 10 Years Que Ans
Multiple Choice Questions
- Among the following equimolar aqueous solutions, identify the one having highest boiling point.
[Mar 08]
(A) Urea
(B) Sucrose
(C) Sodium chloride
(D) Sodium sulphate
2. The addition of non-volatile solute into the pure solvent
[Oct 08]
(A) increases the vapour pressure of solvent
(B) decreases the boiling point of solvent
(C) decreases the freezing point of solvent
(D) increases the freezing point of solvent
3. Which of the following solutions shows maximum depression in freezing point?
(A) ![]()
![]()
(B) ![]()
![]()
(C) ![]()
![]()
(D) ![]()
![]()
4. The temperature at which vapour pressure of a liquid becomes equal to the atmospheric pressure is
(A) melting point
(C) ![]()
![]()
(B) boiling point
(D) ![]()
![]()
[Oct 13]
5. Which of the following is ![]()
![]() a colligative property?
a colligative property?
[Mar 14]
(A) Vapour pressure
(B) Depression in freezing point
(C) Elevation in boiling point
(D) Osmotic pressure
6. Colligative property depends only on in a solution.
[Mar 15]
(A) number of solute particles
(B) number of solvent particles
(C) nature of solute particles
(D) nature of solvent particles
7. The substance ‘ ![]()
![]() ‘, when dissolved in solvent water gave molar mass corresponding to the molecular formula ‘
‘, when dissolved in solvent water gave molar mass corresponding to the molecular formula ‘ ![]()
![]() ‘. The van’t Hoff factor (i) is
‘. The van’t Hoff factor (i) is
[Oct 15]
(A) 3
(B) 0.33
(C) 1.3
(D) 1
8. The determination of molar mass from elevation in boiling point is called as
[Mar 16]
(A) cryoscopy
(B) colorimetry
(C) ebullioscopy
(D) spectroscopy
9. Which of the following ![]()
![]() aqueous solutions will exert the highest osmotic pressure?
aqueous solutions will exert the highest osmotic pressure?
(A) ![]()
![]()
(B) ![]()
![]()
(C) ![]()
![]()
(D) ![]()
![]()
[Mar 18]
10. In calculating osmotic pressure, the concentration of solute is expressed in
(A) molarity
(C) mole fraction
(B) molality
(D) percentage mass
[Mar 22]
Answers:
1.
(D) 2 .
(C) 3 .
(C) 4
4.
(B)
9. (A) 10 . (A)
(B) ![]()
![]() (C)
(C)
- 5 . (A) 6
Solutions:
- The solution having highest number of particles will have highest boling point.

 gives 3 ions on complete dissociation. Hence, among the given equimolar aqueous solutions,
gives 3 ions on complete dissociation. Hence, among the given equimolar aqueous solutions, 
 solution has highest boiling point.
solution has highest boiling point. 
 shows maximum depression in freezing point since 0.5 mole of
shows maximum depression in freezing point since 0.5 mole of 
 will give the highest number of particles, i.e., 1 mole of
will give the highest number of particles, i.e., 1 mole of 
 and 1.5 mole of
and 1.5 mole of 
 ions as compared to other solutions. 0.5 mole of
ions as compared to other solutions. 0.5 mole of 
 gives: 1.5 mole of ions, 1 mole of
gives: 1.5 mole of ions, 1 mole of 
 gives 2 mole of ions and 0.5 mole of
gives 2 mole of ions and 0.5 mole of 
 gives 1.5 mole of ions.
gives 1.5 mole of ions.- van’t Hoff factor (i)
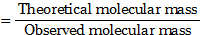
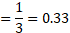
- Colligative properties are proportional to the number of solute particles present in the solution.
Osmotic pressure is a colligative property; hence, ![]()
![]() exerts the highest osmotic pressure.
exerts the highest osmotic pressure.
Calculation: For bcc unit cell, ![]()
![]() .
.
Using formula (i),
Density ![]()
![]()

![]()
![]() .
.
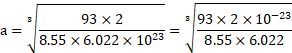

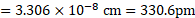
Using formula (ii),
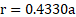
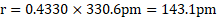

Ans: Radius of niobium atom ( ![]()
![]() ) is
) is ![]()
![]() .
.
- Calculate the number of atoms and unit cells present in

 of Niobium if it forms body centred cubic structure. The density of Niobium is
of Niobium if it forms body centred cubic structure. The density of Niobium is 
 and edge length of unit cell is
and edge length of unit cell is 
 .
.
[July 22]
Solution:
Given: ![]()
![]() Type of unit cell is bcc.
Type of unit cell is bcc.
Density ( ![]()
![]() ) of niobium is
) of niobium is ![]()
![]() ,
,
Edge length (a) ![]()
![]() ,
,
Mass of niobium ![]()
![]()
To find: i. Number of atoms in ![]()
![]() of niobium
of niobium
ii. Number of unit cells in ![]()
![]() of niobium
of niobium
Formulae: i. Number of atoms in ![]()
![]() of metal
of metal ![]()
![]()
ii. Number of unit cells in ![]()
![]() of metal
of metal ![]()
![]()
Calculation: i. For bcc unit cell, ![]()
![]() .
.
Using formula (i),
Number of atoms in ![]()
![]() of niobium
of niobium

ii. Using formula (ii),
Number of unit cells in ![]()
![]() of niobium
of niobium
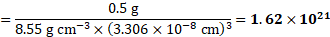
Ans: i. Number of atoms in ![]()
![]() of niobium is
of niobium is ![]()
![]()
ii. Number of unit cells in ![]()
![]() of niobium is
of niobium is ![]()
![]() .
.
Theory Orestions:
2.4 Solubility
- What is the effect of temperature on solubility of a gas in a liquid?
[Oct 15]
Ans:
i. When gases are dissolved in water, the gas molecules in liquid phase are condensed.
ii. The condensation is an exothermic process.
Hence, the solubility of gases in water decreases with increase in temperature.
- State Henry’s law. How does solubility of a gas in water varies with the temperature?
[Oct 13; July 17]
Ans:
i. Statement: Henry’s law states that the solubility of a gas in a liquid is directly proportional to the pressure of the gas over the solution.
ii. ‘ Refer Subtopic 2.4: Q. No. 1.
- State Henry’s law.
Ans: Refer Subtopic 2.4: Q. No. 2.(i).
2.6 Colligative properties of nonelectrolyte solutions
- Define colligative properties.
[Oct 08]
Ans: The physical properties of solutions that depend on the number of solute particles in solutions and not on their nature are called colligative properties.
2.7 Vapour pressure lowering
- Derive the relationship between relative lowering of vapour pressure and molar mass of non-volatile solute.
[Mar 13, 17]
Ans:
i. The relative lowering of vapour pressure is equal to the mole fraction ![]()
![]() of solute in the solution. That is,
of solute in the solution. That is,

ii. The mole fraction of a component of solution is equal to its moles divided by the total moles in the solution. Thus, ![]()
![]()
where, ![]()
![]() and
and ![]()
![]() are the moles of solvent and solute respectively, in the solution
are the moles of solvent and solute respectively, in the solution
iii. In dilute solutions, ![]()
![]() and
and ![]()
![]() . Thus, the mole fraction
. Thus, the mole fraction ![]()
![]() is then given by,
is then given by,
iv. From equations (1) and (2), .
![]()
![]() v. Suppose that we prepare a solution by dissolving
v. Suppose that we prepare a solution by dissolving ![]()
![]() of a solute in
of a solute in ![]()
![]() of solvent. The moles of solute and solvent in the solution are then,
of solvent. The moles of solute and solvent in the solution are then,
![]()
![]() and
and ![]()
![]()
where, ![]()
![]() and
and ![]()
![]() are molar masses of solvent and solute, respectively.
are molar masses of solvent and solute, respectively.
vi. Substitution of equation (4) into equation (3) yields ![]()
![]()
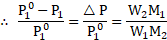
2.8 Boiling point elevation
- What is ‘boiling point’?
[Mar 14]
Ans: The boiling point of liquid is the temperature at which its vapour pressure equals the applied pressure.
- Define ebullioscopic constant.
Write its unit.
[Oct 15]
Ans: Ebullioscopic constant ![]()
![]() ) is the boiling point elevation produced by 1 molal solution.
) is the boiling point elevation produced by 1 molal solution.
Units of ![]()
![]() :
:

- Derive the relation between elevation of boiling point and molar mass of solute.
[Mar 18] OR
Derive the mathematical expression between molar mass of a non-volatile solute and elevation of boiling point.
[Mar 20]
OR
How will you determine molar mass of non volatile solute by elevation of boiling point?
[Mar 23]
Ans:
i. The boiling point elevation is directly proportional to the molality of the solution.
Thus,
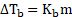
ii. Suppose we prepare a solution by dissolving ![]()
![]()
![]()
![]() of solute in
of solute in ![]()
![]() of solvent.
of solvent.
Moles of solute in ![]()
![]() of solvent
of solvent ![]()
![]()
where, ![]()
![]() is the molar mass of solute.
is the molar mass of solute.
Mass of solvent ![]()
![]()
iii. The molality is expressed as,
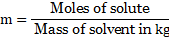

Std. XII Sci: Board Questions with Solutions (Chemistry)
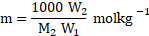
iv. Substituting equation (2)
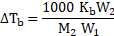
Hence, ![]()
![]()
- Define ebullioscopic constant.
[Oct 09; July 22]
Ans: Refer Subtopic 2.8: Q. No. 2. (Definition)
2.9 Depression in freezing point
- Define boiling point. Write the formula to determine molar mass of a solute using freezing point depression method. [Mar 16]
Ans:
i. Refer Subtopic 2.8: Q. No. 1.
ii. The formula to determine molar mass of a solute using freezing point depression method is
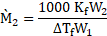
- Define: Freezing point. OR
[July 16]
What is freezing point of a liquid? [July 18]
Ans: The freezing point of a liquid is the temperature at which liquid and solid are in equilibrium and the two phases have the same vapour pressure.
- Define :Cryoscopic constant. [Oct 14; July 18]
Ans: Cryoscopic constant is the depression in freezing point produced by 1 molal solution of a non-volatile solute.
- Write mathematical expression between cryoscopic constant and molar mass of solute.
[July 19]
Ans: Refer Subtopic 2.9: Q. No. 1.(ii).
- Write the SI unit of cryoscopic constant.
[Mar 23]
Ans: The SI unit of cryoscopic constant is ![]()
![]() .
.
2.10 Osmotic pressure
- Define semipermeable membrane. [Mar 18]
Ans: Semipermeable membrane is a film such as cellophane which has pores large enough to allow the solvent molecules to pass through them.
- Define osmotic pressure.
[Mar 20; July 17, 22]
Ans: The hydrostatic pressure (on the side of solution) that stops osmosis is called an osmotic pressure of the solution.
OR
The excess of pressure on the side of the solution that stops the net flow of solvent into the solution through a semipermeable membrane is called osmotic pressure.
- Define the following terms:
i. Isotonic solution [Oct 13; Mar 09, 19, 22]
ii. Hypertonic solution [Mar 19]
iii. Hypotonic solution [Mar 19]
Ans:
i. Two or more solutions having the same osmotic pressure are said to be isotonic solutions.
e.g. For example, ![]()
![]() urea solution and
urea solution and ![]()
![]() sucrose solution are isotonic because their osmotic pressures are equal.
sucrose solution are isotonic because their osmotic pressures are equal.
ii. If two solutions have unequal osmotic pressures, the more concentrated solution with higher osmotic pressure is said to be hypertonic solution.
- e.g. For ‘example, if osmotic pressure of sucrose solution is higher than that of urea solution, the sucrose solution is hypertonic to urea solution.
iii. If two solutions have unequal osmotic pressures, the more dilute solution exhibiting lower osmotic pressure is said to be hypotonic solution.
e.g. For example, if osmotic pressure of sucrose solution is higher than that of urea solution, the urea solution is hypotonic to sucrose solution.
- Define osmosis. [Mar 13, 22, 23; July 16, 19]
Ans: The net spontaneous flow of solvent molecules into the solution or from more dilute solution to more concentrated solution through a semipermeable membrane is called osmosis.
- Derive an expression to calculate molar mass of non volatile solute by osmotic pressure measurement.
[Mar 22]
Ans:
i. For very dilute solutions, the osmotic pressure follows the equation,

ii. If the mass of solute in ![]()
![]() litres of solution is
litres of solution is ![]()
![]() and its molar mass is
and its molar mass is ![]()
![]() , then
, then ![]()
![]() .
.
Substituting the value of ![]()
![]() in equation (1), we get
in equation (1), we get ![]()
![]()
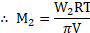
This formula can be used for the calculation of molar mass of a nonionic solute (i.e., nonelectrolyte), by osmotic pressure ‘ measurement.
2.11 Colligative properties of electrolytes
- Define van’t Hoff factor. How is it related to the degree of dissociation?
[Oct 14]
Ans:
i. van’t Hoff factor (i) is defined as the ratio of colligative property of a solution of electrolyte divided by the colligative property of nonelectrolyte solution of the same concentration.
ii. The van’t Hoff factor is related to degree of ionization as follows:
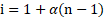
or ![]()
![]()
where, ![]()
![]() Degree of ionization/dissociation
Degree of ionization/dissociation
![]()
![]() van’t Hoff factor
van’t Hoff factor
![]()
![]() Moles of ions obtained from ionization of 1 mole of electrolyte.
Moles of ions obtained from ionization of 1 mole of electrolyte.
Numerical
2.4 Solubility
- What is the concentration of dissolved oxygen at

 under pressure of one atmosphere if partial pressure of oxygen at
under pressure of one atmosphere if partial pressure of oxygen at 
 is
is 
 ? (Henry’s law constant for oxygen
? (Henry’s law constant for oxygen 
 )
)
Solution: Henry’s law constant ![]()
![]() ,
,
[Mar 20]
Partial pressure of the gas ![]()
![]()
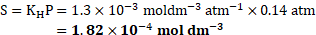
Ans: Concentration of dissolved oxygen is ![]()
![]() .
.

water at same temperature and partial pressure of ![]()
![]() ?
?
Solution: Henry’s law constant ![]()
![]() ,
,
Pressure of the gas ![]()
![]()
Solubility ![]()
![]()
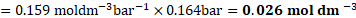
Ans: Sólubility of ![]()
![]() gas in water is
gas in water is ![]()
![]() .
.
2.7 Vapour pressure lowering
- The vapour pressure of pure benzene is

 of non-volatile solute is added to
of non-volatile solute is added to 
 of benzene, the vapour pressure of solution is
of benzene, the vapour pressure of solution is 
 of
of 
 . Calculate molar mass of solute
. Calculate molar mass of solute 
 .
.
[Mar 16]
Solution:
Given: ![]()
![]() Vapour pressure of pure benzene
Vapour pressure of pure benzene ![]()
![]()
Molar mass of benzene ![]()
![]()
Mass of non-volatile solute ![]()
![]()
Mass of benzene ![]()
![]()
To find: ![]()
![]() Molar mass of non-volatile solute
Molar mass of non-volatile solute ![]()
![]()
Formula: ![]()
![]()
Calculation: Using the formula,
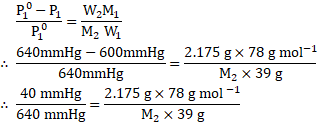
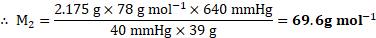
Ans: The molar mass of the non-volatile solute is ![]()
![]() .
.
- Calculate the mole fraction of solute, if the vapour pressure of pure benezene at certain temperature is

 and vapour pressure of solution of a solute in benzene is
and vapour pressure of solution of a solute in benzene is 
 .
.
Solution:
Given: ![]()
![]() Vapour pressure of pure benzene
Vapour pressure of pure benzene ![]()
![]()
Vapour pressure of solution ![]()
![]()
To find: , Mole fraction of solute ![]()
![]()
Formula: ![]()
![]()
Calculation: Using the formula,
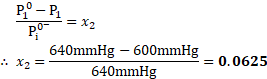
Ans: The mole fraction of solute is ![]()
![]() .
.
2.8 – Boiling point elevation
- A solution containing

 of camphor (molar mass
of camphor (molar mass 
 ) in
) in 
 of acetone (boiling point
of acetone (boiling point 
 ) boils at
) boils at 
 . A solution of
. A solution of 
 of unknown compound in the same weight of acetone boils at
of unknown compound in the same weight of acetone boils at 
 . Calculate the molar mass of the unknown compound.
. Calculate the molar mass of the unknown compound.
[Oct 14]
Solution:
Given: ![]()
![]() Mass of camphor
Mass of camphor ![]()
![]() , Molar mass of camphor
, Molar mass of camphor ![]()
![]()
Mass̃ of acetone ![]()
![]() , Mass of unknown compound
, Mass of unknown compound ![]()
![]()
To find: ![]()
![]() Molar mass of unknown compound
Molar mass of unknown compound ![]()
![]()
Formula: ![]()
![]()
Cálculation: For solution of camphor in acetone,

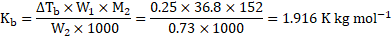
For solution of unknown compound in acetone,

Using the formula,

Ans:- The molar mass of the unknown compound is ![]()
![]() .
.

 of sulphur is dissolved in
of sulphur is dissolved in 
 of
of 
 . This solution boils at
. This solution boils at 
 . What is molecular formula of sulphur in solution? The boiling point of
. What is molecular formula of sulphur in solution? The boiling point of 
 is
is 
 .
.
Solution:
[Given that ![]()
![]() for
for ![]()
![]() and atomic mass of
and atomic mass of ![]()
![]() .]
.]
Given: ![]()
![]() Mass of sulphur
Mass of sulphur ![]()
![]() , Mass of solvent
, Mass of solvent ![]()
![]()
Boiling point of solution ![]()
![]() , Boiling point of pure solvent
, Boiling point of pure solvent ![]()
![]()
Molal elevation constant ![]()
![]()
Atomic mass of sulphur ![]()
![]()
To find: ![]()
![]() Molecular formula of sulphur in solution
Molecular formula of sulphur in solution
Formula: ![]()
![]()
Calculation: For solution of sulphur in ![]()
![]() (solvent),
(solvent),

From formula,
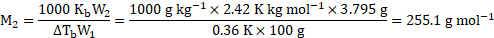
Now, atomic mass of ![]()
![]()
![]()
![]() Number of Sulphur atoms in a molecule
Number of Sulphur atoms in a molecule ![]()
![]()
![]()
![]() Molecular formula of sulphur in
Molecular formula of sulphur in ![]()
![]()
Ans: Molecular formula of sulphur in ![]()
![]() ‘solution is
‘solution is ![]()
![]() .
.
- The boiling point of benzene is

 . When 1.80 gram of non-volatile solute was dissolved in 90 gram of benzene, the boiling point is raised to
. When 1.80 gram of non-volatile solute was dissolved in 90 gram of benzene, the boiling point is raised to 
 . Calculate the molar mass of solute.
. Calculate the molar mass of solute. 
 for benzene
for benzene 

[Mar 17]
Solution:
Given:
Boiling point of benzene ![]()
![]()
Boiling point of solution ![]()
![]()
Mass of non-volatile solute ![]()
![]()
Mass of benzene ![]()
![]()
To find: ![]()
![]() Molar mass of solute
Molar mass of solute ![]()
![]()
Formula: ![]()
![]()
Calculation: For solution of non-volatile solute and benzene,
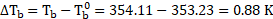
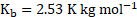
Now, using formula and substituting values,
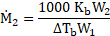
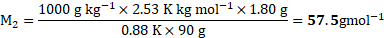
Ans: The molar mass of non-volatile solute is ![]()
![]() .
.
- The normal boiling point of ethyl acetate is

 . A solution of
. A solution of 
 of non-volatile solute in
of non-volatile solute in 
 of ethyl acetate boils at
of ethyl acetate boils at 
 . Evaluate the molar mass of solute if
. Evaluate the molar mass of solute if 
 for ethyl acetate is
for ethyl acetate is 
 .
.
[July 22]
Solution:
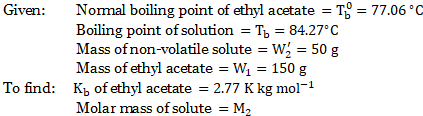
Formula: ![]()
![]()
Calculation: For solution of non-volatile solute and ethyl acetate,
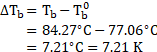

Now, using formula and substituting values,

Ans: The molar mass of non-volatile solute is ![]()
![]() .
.
2.9 Depression in freezing point

 of urea when dissolved in
of urea when dissolved in 
 of a solvent, decreases freezing point of the solvent by
of a solvent, decreases freezing point of the solvent by 
 of another non-electrolyte solute when dissolved in
of another non-electrolyte solute when dissolved in 
 of the same solvent depresses the freezing point by
of the same solvent depresses the freezing point by 
 . Calculate the molar mass of the another solute.
. Calculate the molar mass of the another solute.
[Given: molar mass of urea ![]()
![]() ]
]
Solution:
For solution containing urea:
Mass of urea ![]()
![]() , Molar mass of urea
, Molar mass of urea ![]()
![]()
Mass of solvent ![]()
![]()
Depression in freezing point of solution containing urea ![]()
![]()
For solution containing unknown solute:
Mass of unknown solute ![]()
![]()
Mass of solvent ![]()
![]()
Depression in freezing point of solution containing unknown solute ![]()
![]()
To find: ![]()
![]() Molar mass of unknown solute
Molar mass of unknown solute ![]()
![]()
Formula: ![]()
![]()
Calculation: Using the formula and rearranging, we get,

From equations (1) and (2),
( ![]()
![]() Since the solvent is same in both solutions, the value of
Since the solvent is same in both solutions, the value of ![]()
![]() will be same for both the solutions.)
will be same for both the solutions.)
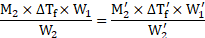
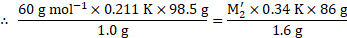
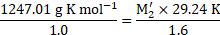

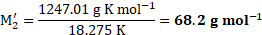
Ans: The molar mass of the unknown solute is ![]()
![]() .
.
- The freezing point of pure benzene is

 . Calculate the freezing point of the solution when
. Calculate the freezing point of the solution when 
 of a solute having molecular weight
of a solute having molecular weight 
 is added to
is added to 
 of benzene.
of benzene.
![]()
![]() of benzene
of benzene ![]()
![]()
Solution:
Freezing point of pure solvent ![]()
![]()
Mass of solute ![]()
![]()
Molar mass of solute ![]()
![]()
Mass of solvent ![]()
![]()
Molal depression constant ![]()
![]()
To find: ![]()
![]() Freezing point of solution
Freezing point of solution ![]()
![]()
Formula: ![]()
![]()
Calculation: Rearranging the formula,
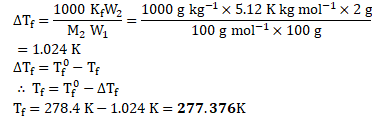
Ans: Freezing point of the solution is ![]()
![]() .
.

 aqueous solution of cane sugar has freezing point of
aqueous solution of cane sugar has freezing point of 
 . Calculate freezing point of
. Calculate freezing point of 
 glucose solution. [Molar mass of cane sugar
glucose solution. [Molar mass of cane sugar 
 ]
]
[July 22]
Solution:
Given: ![]()
![]() Percentage by mass of cane sugar solution
Percentage by mass of cane sugar solution ![]()
![]()
Percentage by mass of glucose solution ![]()
![]() ,
,
Freezing point of cane sugar solution ![]()
![]()
Molar mass of cane sugar ![]()
![]()
To find: . Freezing point of glucose solution
Formula: ![]()
![]()
Calculation: ![]()
![]() solution (by mass) of cane sugar means that mass of cane sugar
solution (by mass) of cane sugar means that mass of cane sugar ![]()
![]() , and mass of solvent
, and mass of solvent ![]()
![]() .
.
![]()
![]() glucose solution means that mass of glucose
glucose solution means that mass of glucose ![]()
![]() , and mass of solvent
, and mass of solvent ![]()
![]() .
.
Molar mass of glucose ![]()
![]()
![]()
![]() for cane sugar solution
for cane sugar solution ![]()
![]()
Now, using the formula,
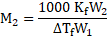
Rearranging the formula, we get
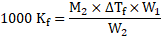

From equations (1) and (2),


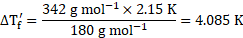
![]()
![]() Freezing point of glucose solution
Freezing point of glucose solution ![]()
![]()
Ans: Freezing point of glucose solution is ![]()
![]() .
.
Alternate method:
Formula: ![]()
![]()
Calculation: ![]()
![]() solution (by mass) of cane sugar means that mass of cane sugar
solution (by mass) of cane sugar means that mass of cane sugar ![]()
![]() , and mass of solvent
, and mass of solvent ![]()
![]() .
.
![]()
![]() for cane sugar solution
for cane sugar solution ![]()
![]()
Now, using formula,
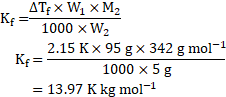
Now, ![]()
![]() glucose solution means that mass of glucose
glucose solution means that mass of glucose ![]()
![]() , and mass of solvent
, and mass of solvent ![]()
![]() .
.
Molar mass of glucose ![]()
![]()
Using formula,
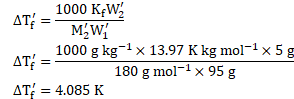
![]()
![]() Freezing point of glucose solution
Freezing point of glucose solution ![]()
![]()
Ans: Freezing point of glucose solution is ![]()
![]() .
.
[Note: In the above problem, the solvent is water and therefore the ![]()
![]() value should be
value should be ![]()
![]() and based on this value,
and based on this value, ![]()
![]() (glucose) will be
(glucose) will be ![]()
![]() and
and ![]()
![]() will be
will be ![]()
![]() . However; we find that on using the alternate method, we get the value of
. However; we find that on using the alternate method, we get the value of ![]()
![]() as
as ![]()
![]() .]
.]
2.10 Osmotic pressure

 of a substance dissolved in
of a substance dissolved in 
 of water gave an osmotic pressure of 1.331 atmospheres at
of water gave an osmotic pressure of 1.331 atmospheres at 
 . Calculate the molecular weight of the substance.
. Calculate the molecular weight of the substance.
(Given: ![]()
![]() ).
).
[Mar 09]
Solution:
Given:
Volume ![]()
![]()
Mass of substance ![]()
![]()
Osmotic pressure ![]()
![]()
Temperature ![]()
![]()
To find: ![]()
![]() Molecular weight
Molecular weight ![]()
![]()
Formula: ![]()
![]()
Calculation: From formula,

Ans: The molecular weight of the solute is ![]()
![]() .
.
- A solution of a substance having mass

 has the osmotic pressure of
has the osmotic pressure of 
 at
at 
 .
.
Calculate the molar mass of the substance used. [Volume ![]()
![]() ] [Mar 17]
] [Mar 17]
Solution:
Given: ![]()
![]() Volume
Volume ![]()
![]()
Mass of substance ![]()
![]()
Osmotic pressure ![]()
![]()
![]()
![]() or
or ![]()
![]()
Temperature ![]()
![]()
To find: Molar mass ![]()
![]()
Formula: ![]()
![]()
Calculation: From formula,
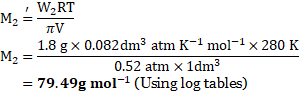

Ans: The molar mass of a solute is ![]()
![]() .
.
- An organic substance

 is dissolved in
is dissolved in 
 of water. Its osmotic pressure at
of water. Its osmotic pressure at 
 was found to be
was found to be 
 . If
. If 
 , calculate the mass of the solute.
, calculate the mass of the solute.
[July 17]
Solution:
Given: ![]()
![]() Volume
Volume ![]()
![]()
Molar mass of the substance ![]()
![]()
Osmotic pressure ![]()
![]()
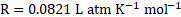
Temperature ![]()
![]()
To find:
Mass of the solute ![]()
![]()
Formula:
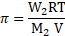
Calculation: From formula,
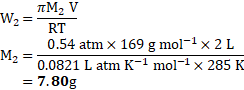
Ans: The mass of the solute is ![]()
![]() .
.
2.11 Colligative properties of electrolytes
- Calculate the amount of

 (van’t Hoff factor
(van’t Hoff factor 
 ) dissolved in
) dissolved in 
 solution so that its osmotic pressure at
solution so that its osmotic pressure at 
 is 0.75 atmosphere.
is 0.75 atmosphere.
Solution:
[Given: Molar mass of ![]()
![]() is
is ![]()
![]() ]
]
Given: . Osmotic pressure of solution ![]()
![]()
Volume of solution ![]()
![]() , van’t Hoff factor
, van’t Hoff factor ![]()
![]()
Temperature ![]()
![]() , Molar mass of solute,
, Molar mass of solute, ![]()
![]()
To find: ![]()
![]()
Formula: ![]()
![]()
Calculation: Using formula, ![]()
![]()

Ans: Amount of ![]()
![]() in the solution is
in the solution is ![]()
![]() .
.