Chp 1 solid state board exam questions
2019-2023 HSC Board Exam Paper Questions and Answer
Multiple Choice Otestions:
- In body centred cubic structure, the space occupied is about
(A)

(B)

(C) .

(D)

[Mar 13]
- To prepare n-type semiconductor, the impurity to be added to silicon should have the following number of valence electrons:
[Mar 14]
(A) 2
(B) 3
(C) 4
(D) 5
- The major binding force in diamond is
(A) covalent bond
[Oct 14]
(B) ionic bond
(C) metallic bond
(D) coordinate covalent bond
- p-type semi-conductors, are made by mixing silicon with impurities of
[Mar 15]
(A) germanium
(B) boron
(C) arsenic
(D) antimony
- An ionic compound crystallises in FCC type structure with ‘

 ‘ ions at the centre of each face and ‘
‘ ions at the centre of each face and ‘ 
 ‘ ions occupying corners of the cube. The formula of compound is
‘ ions occupying corners of the cube. The formula of compound is
[Mar 17]
(A) ![]()
(B)
(C)
(D)
- Number of types of orthorhombic-unit cell is
(A) 7
(B) 3
(C) 4
(D) 2 - The number of atoms per unit cell of body centred cube is:
[Mar 20]
(A) 1
(B) 2
(C) 4
(D) 6
- The co-ordination number of atoms in body centred cubic structure (bcc) is
[Mar 22]
(A) 4
(B) 6
(C) 8
(D) 12
- The CORRECT relation between edge length and radius of an atom in simple cubic lattice is
[July 22]
(A) ![]()
![]()
(B) ![]()
![]()
(C) ![]()
![]()
(D) ![]()
![]()
- The relation between radius of sphere and edge length in body centered cubic lattice is given by formula:
[Mar 23]
(A) ![]()
![]()
(B) ![]()
![]()
(C) ![]()
![]()
(D) ![]()
![]()
Answers:
| 1. | (D) | 2. | (D) | 3. | (A) | 4. | (B) |
|---|---|---|---|---|---|---|---|
| 5. | (B) | 6. | (C) | 7. | (B) | 8. | (C) |
| 9. | (C) | 10. | (C) |
Solution:
- ‘As ‘

 ‘ ions are present at the face centres of the 6 faces of the cube, the number of ions of ‘
‘ ions are present at the face centres of the 6 faces of the cube, the number of ions of ‘ 
 ‘ in the unit cell
‘ in the unit cell 

As ‘ ![]()
![]() ‘ is present at the 8 corners of the cube, number of ions of
‘ is present at the 8 corners of the cube, number of ions of ![]()
![]() in the unit cell
in the unit cell
![]()
![]() Ratio of atoms
Ratio of atoms ![]()
![]() .
.
The formula of the compound is ![]()
![]() .
.
Theur Orestions
1.2 Types of solids
- Distinguish between crystalline solids and amorphous solids.
[Mar 13, 14, 17, 19]
Ans:
| i. |
|
| ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| ii. |
|
| ||||||||
| iii. |
|
| ||||||||
| iv. |
|
| ||||||||
| e.g. | Ice, NaCl, etc. |
|
1.3 Classification of crystalline solids
- Classify the following molecular solids into
different types:
| i. | ii. ‘ |
|---|---|
| iii. Solid ice | iv: |
[July 18]
Ans:
i. ![]()
![]() – Polar molecular solid
– Polar molecular solid
ii. ![]()
![]() – Non-polar molecular solid
– Non-polar molecular solid
iii. Solid ice – Hydrogen bonded molecular solid
iv. ![]()
![]() – Polar molecular solid
– Polar molecular solid
- Classify the following solids into different types:
i. Silver
iii. Diamond
ii. ![]()
![]()
iv. ![]()
![]()
Ans:
i. Silver-Metallic solid
ii. ![]()
![]() – Molecular solid
– Molecular solid
iii. Diamond – Covalent network solid
iv. ![]()
![]() – Ionic solid
– Ionic solid
1.5 Cubic system
- A face centred cube (fcc) consists of how many atoms? Explain.
[July 16] OR
[Mar 20]
Calculate the number of atoms in a unit cell of a metal crystallising in face centred cubic structure.
[July 17]
Ans:
i. A face-centred cubic (fcc) unit cell has particles (atoms)’ at the eight corners plus particles (atoms) at the centre of its six faces.
ii. Each particle present at the corner of a given unit cell is shared with seven other neighbouring unit cells. As a result, its contribution to the given unit cell is only ![]()
![]() .
.
Thus, the number of atoms present at corners per unit cell ![]()
![]() corner atoms
corner atoms ![]()
![]() atom per unit – cell
atom per unit – cell ![]()
![]()
iii. Each particle at the centre of the six faces is shared with one neighbouring cube. Thus, ![]()
![]() of each face particle belongs to the given unit cell.
of each face particle belongs to the given unit cell.
Thus, the number of atoms present at faces per unit cell ![]()
![]() atoms at the faces
atoms at the faces ![]()
![]() atom per unit cell
atom per unit cell ![]()
![]()
Therefore, the total number of atoms per unit cell ![]()
![]()
Thus, fcc unit cell has total of 4 atoms per unit cell.
1.6 Packing of particles in crystal lattice
- What is the ratio of octahedral holes to the number of anions in hexagonal closed packed structure?
[Mar 19]
Ans: The ratio of octahedral holes to the number of anions in hexagonal closed packed structure is ![]()
![]() .
.
1.7 Packing efficiency
- Calculate the percentage efficiency of packing in case of simple cubic cell. [Mar 17]
Ans: Packing efficiency of metal crystal in simple cubic lattice:
Step 1: Radius of sphere:
In simple cubic unit cell, particles (spheres) are at the corners and touch each other along the edge.
Thus, ![]()
![]() or
or ![]()
![]()
where, ‘ ![]()
![]() ‘ is the radius of atom and ‘
‘ is the radius of atom and ‘ ![]()
![]() ‘ is the length of unit cell edge.
‘ is the length of unit cell edge.
Step 2: Volume of sphere:
Volume of a sphere ![]()
![]() .
.
Substitution for ![]()
![]() from equation (1) gives:
from equation (1) gives:
Volume of one particle ![]()
![]()
![]()
Step 3: Total volume of particles:
Because simple cubic unit cell contains only one particle, volume occupied by particle in unit cell
![]()
Step 4: Packing efficiency:
Packing efficiency
![]()
![]()
![]()
Thus, in simple cubic lattice, ![]()
![]() of total space is occupied by particles.
of total space is occupied by particles.
- Give the relation between radius of atom and edge length in body centered cubic crystal.
[July 19]
Ans: The relation between radius of atom and edge length in body centered cubic crystal is:
![]()
1.8 Crystal defects or imperfections
- What are Schottky defect and Frenkel defect?
[Oct 13]
Ans:
i. Schottky defect:
a. Schottky defect is formed in an ionic solid, when equal number of cations and anions are missing from their regular positions in the crystal lattice thereby creating vacancies. Such a paired cationanion vacancy defect is a Schottky defect.
b. This defect is found in ionic crystals such as ![]()
![]() and
and ![]()
![]() .
.
ii. Frenkel defect:
a. Frenkel defect arises when an ion of an ionic compound is missing from its regular lattice site and occupies interstitial position between lattice points. The cations are usually smaller than anions. Therefore, the cations occupy interstitial sites. Frenkel defect can be regarded as the combination of vacancy defect and interstitial defect.
b. This defect is found in ionic crystals like ![]()
![]() ,
, ![]()
![]() and
and ![]()
![]() .
.
- Explain impurity defect in stainless steel with diagram.
[Mar 15]
Ans: In interstitial impurity defect, the impurity atoms occupy interstitial spaces of lattice structure.
e.g.: Stainless steel
In stainless steel, ![]()
![]() atoms occupy normal lattice sites. The carbon atoms are present at interstitial spaces, as shown in the figure.
atoms occupy normal lattice sites. The carbon atoms are present at interstitial spaces, as shown in the figure.
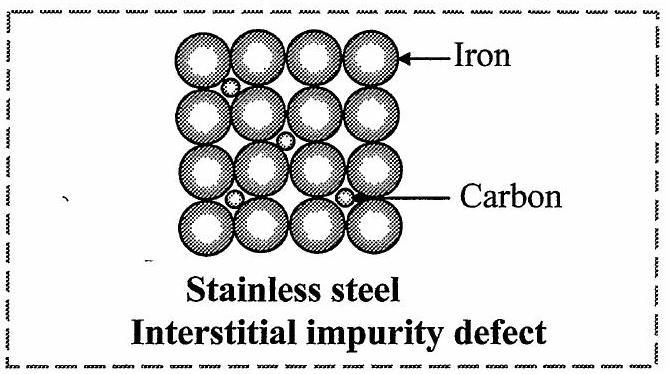
- What is Schottky defect?
Ans: Refer Subtopic 1.8: Q.No.1.(i)
[July 19]
- Write the consequences of Schottky defect with reasons.
[Mar 22]
Ans: Consequences of Schottky defect:
i. As the number of ions decreases, mass decreases. However, volume remains unchanged. Hence, the density of a substance decreases.
ii. The number of missing cations and anions is equal. Hence, the electrical neutrality of the compound is preserved.
- Explain metal deficiency defect with example.
[Mar 22]
Ans: Metal deficiency defect:
i. This defect is possible only in compounds of metals that show variable oxidation states.
ii. In some crystals, positive metal ions are missing from their original lattice sites. The extra negative charge is balanced by the presence of cation of the same metal with higher oxidation state than that of missing cation.
e.g. In the compound ![]()
![]() , one
, one ![]()
![]() ion is missing creating a vacancy at its lattice site. The deficiency of two positive charges is made up by the presence of two
ion is missing creating a vacancy at its lattice site. The deficiency of two positive charges is made up by the presence of two ![]()
![]() ions at the other lattice sites of
ions at the other lattice sites of ![]()
![]() ions. The composition of
ions. The composition of ![]()
![]() then becomes
then becomes ![]()
![]() .
.
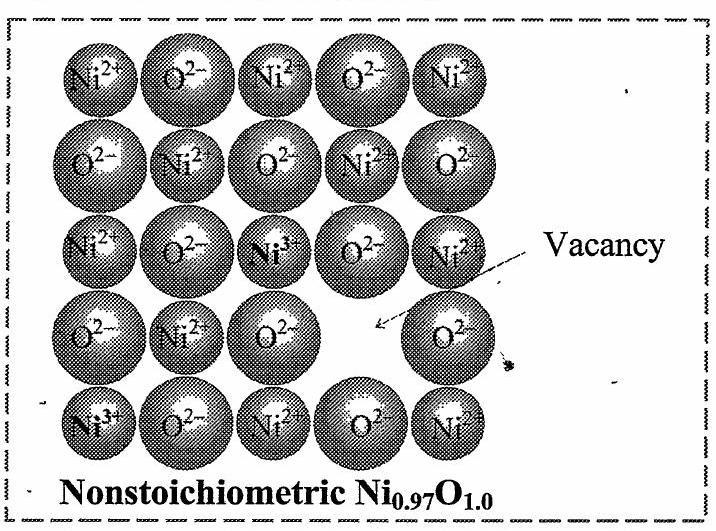
- Distinguish between Schottky and Frenkel defect.
[July 22]
Ans: Differences between Schottky and Frenkel defect:
| No. | Schottky defect | Frenkel defect | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| i. |
|
| ||||||||||||||||||
| ii. |
|
| ||||||||||||||||||
| iii. |
|
| ||||||||||||||||||
| iv. |
|
| ||||||||||||||||||
| v. |
|
| ||||||||||||||||||
| vi. |
|
|
(Any four distinguishing points)
- Explain the following terms:
[Mar 23]
i. Substitutional impurity defect
ii. Interstitial impurity defect
Ans:
i. Substitutional impurity defect:
In this defect, the foreign atoms are found at the lattice sites in place of host atoms. The regular atoms are displaced from their lattice sites by impurity atoms.
e.g.: Brass :.:
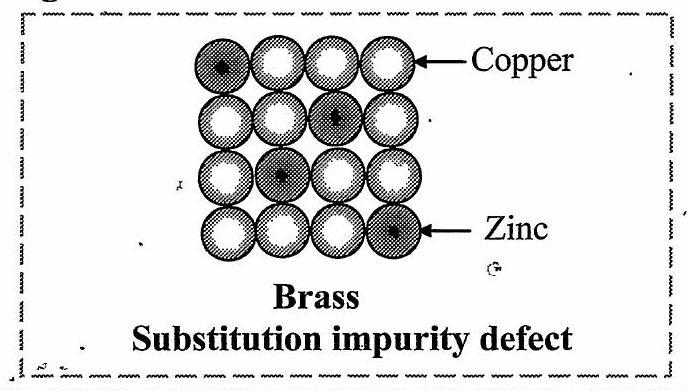
Std. XII Sci: Board Questions with Solutions (Chemistry)
ii. Interstitial impurity defect:
In this defect, the impurity atoms occupy interstitial spaces of lattice structure.
e.g.: Steel
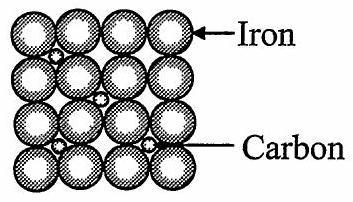
Stainless steel
Interstitial impurity defect
1.10 Magnetic properties of solids
- What is ferromagnetism?
[Mar 16]
Ans: The substances containing large number of unpaired electrons-are attracted strongly by magnetic field. Such substances are called as ferromagnetic substances. The property thus exhibited is called ferromagnetism.
Numerical
1.5 Cubic system
- Face centred cubic crystal lattice of copper has density of

 . Calculate the volume of the unit cell. Solution:
. Calculate the volume of the unit cell. Solution:
[Given: Molar mass of copper is ![]()
![]() and Avogadro number
and Avogadro number ![]()
![]() is
is ![]()
![]() .] [Mar 14]
.] [Mar 14]
Given: ![]()
![]() Density
Density ![]()
![]() , Molar mass of copper
, Molar mass of copper ![]()
![]()
To find: Volume of the unit cell
Formula: ![]()
![]() Density
Density ![]()
![]()
Calculation: For fcc unit cell, ![]()
![]() .
.
From formula,
Volume of unit cell, ![]()
![]()
![]()
Ans: The volume of the unit cell is ![]()
![]() .
.
- A unit cell of iron crystal has edge length

 and density
and density 
 . Find the number of atoms per unit cell and type of the crystal lattice.
. Find the number of atoms per unit cell and type of the crystal lattice.
[Given: Molar mass of iron ![]()
![]() ,
,
Avogadro’s number ![]()
![]() ]
]
[Oct 14]
Solution:
Given: ![]()
![]() Edge length (a)
Edge length (a) ![]()
![]() , Atomic mass
, Atomic mass ![]()
![]() , Density
, Density ![]()
![]()
To find: ![]()
![]() Type of crystal lattice
Type of crystal lattice
Formula: ![]()
![]() Density
Density ![]()
![]()
Calculation: From formula, Density, ![]()
![]()
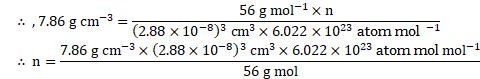
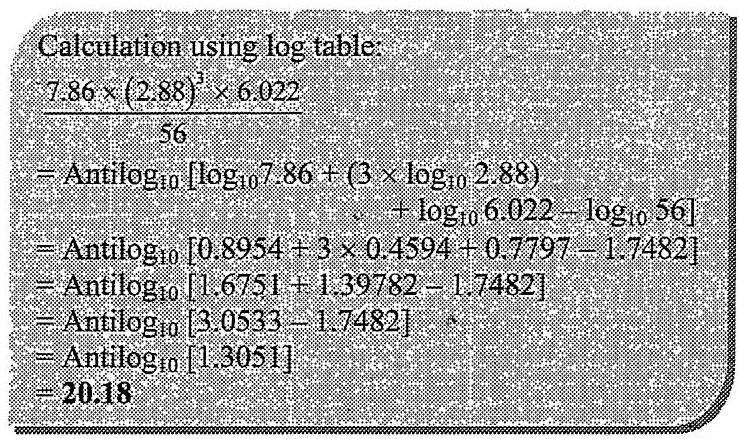
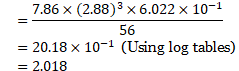
![]()
![]() Number of atoms in unit cell
Number of atoms in unit cell ![]()
![]()
Since unit cell contains 2 atoms, it has body-centred cubic (bcc) structure.
Ans: The type of crystal lattice is body-centred cubic (bcc) lattice.
- Silver crystallises in fcc (face-centred cubic crystal) structure. The edge length of the unit cell is found to be

 . Calculate density of the unit cell. [Given: Molar mass of silver is
. Calculate density of the unit cell. [Given: Molar mass of silver is 
 ] [Oct 15]
] [Oct 15]
Solution:
Given:
To find:
Edge length (a) ![]()
![]() , Molar mass
, Molar mass ![]()
![]()
Density ![]()
![]()
Formula:
![]()
Calculation: ![]()
![]() For an fcc lattice, number of atoms per unit cell is 4.
For an fcc lattice, number of atoms per unit cell is 4.
![]()
From formula, Density, ![]()
![]()
![]()
Ans: Density of silver is ![]()
![]() .
.
- Silver crystallises in fec structure. If density of silver is

 , calculate the volume of unit cell.
, calculate the volume of unit cell.
[Mar 16]
Solution:
Given: ![]()
![]() Density
Density ![]()
![]()
To find: ![]()
![]() Volume of the unit cell
Volume of the unit cell
Formula:
Density ![]()
![]()
Calculation: For fcc unit cell, ![]()
![]() .
.
From formula, Volume of unit cell, ![]()
![]()
![]()
Ans: The volume of the unit cell is ![]()
![]() .
.
- Determine the density of cesium chloride which crystallizes in bec type structure with the edge length

 . The atomic masses of
. The atomic masses of 
 and
and 
 are 133 and 35.5 respectively.
are 133 and 35.5 respectively.
Solution:
Given: ![]()
![]() Edge length (a)
Edge length (a) ![]()
![]() , Molar mass
, Molar mass ![]()
![]()
To find: ![]()
![]() Density
Density ![]()
![]()
Formulae: i. ![]()
![]() Mass of one molecule
Mass of one molecule ![]()
![]()
ii. Volume of unit cell ![]()
![]()
iii. Density ![]()
![]()
Calculation: In the bcc type unit cell of ![]()
![]() , there is one
, there is one ![]()
![]() ion at the body centre position and
ion at the body centre position and ![]()
![]() ions are at the 8 corners.
ions are at the 8 corners.
![]()
![]() Number of
Number of ![]()
![]() in unit cell
in unit cell ![]()
![]()
Number of ![]()
![]() in unit cell
in unit cell ![]()
![]()
Hence, the unit cell contains one ![]()
![]() molecule.
molecule.
Std. XII Sci. Board Questions with Solutions (Chemistry)
Mass of one ![]()
![]() molecule
molecule ![]()
![]()
![]()
![]()
![]() Mass of unit cell
Mass of unit cell ![]()
![]()
Volume of unit cell ![]()
![]()
![]()
![]() Density
Density ![]()
![]()
Ans: Density of cesium chloride is ![]()
![]() .
.
![]()
- A metal crystallises into two cubic faces namely face centred (fcc) and body centred (bcc), whose unit cell edge lengths are 3.5

 and 3.0
and 3.0 
 respectively. Find the ratio of the densities of fec and bcc. [July 17]
respectively. Find the ratio of the densities of fec and bcc. [July 17]
Solution:
Given: ![]()
![]() Edge length of fcc unit cell
Edge length of fcc unit cell ![]()
![]() Edge length of bcc unit cell
Edge length of bcc unit cell ![]()
![]()
To find: ![]()
![]() Ratio of densities of fcc and
Ratio of densities of fcc and ![]()
![]()
Formula: ![]()
![]() Density of unit cell
Density of unit cell ![]()
![]()
Calculation: -For fcc unit cell, ![]()
![]() .
.
Density of fcc unit cell ![]()
![]()
For bcc unit cell, ![]()
![]() .
.
Density of bcc unit cell ![]()
![]() ,
,
![]()
![]() Ratio of densities of fcc and bcc unit cell is,
Ratio of densities of fcc and bcc unit cell is,
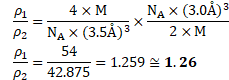
Ans: The ratio of densities of fcc and bcc structures is ![]()
![]()
- The density of iron crystal is

 . If the edge length of unit cell is
. If the edge length of unit cell is 
 and atomic mass is
and atomic mass is 
 , find the number of atoms in the unit cell.
, find the number of atoms in the unit cell.
[Given: Avogadro’s number ![]()
![]() ]
]
Solution:
Given: ![]()
![]() Edge length (a)
Edge length (a) ![]()
![]() , Atomic mass
, Atomic mass ![]()
![]() , Density
, Density ![]()
![]()
To find: ![]()
![]() Number of atoms in the unit cell
Number of atoms in the unit cell
Formula: ![]()
![]() Density
Density ![]()
![]()
Calculation: From formula,
Density, ![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]() Number of atoms in unit cell
Number of atoms in unit cell ![]()
![]()
Ans: The number of atoms in the unit cell is 2.
- The density of silver having atomic mass

 is
is 
 . If the edge, length of cubic unit cell is
. If the edge, length of cubic unit cell is 
 , find the number of silver atoms in the unit cell.
, find the number of silver atoms in the unit cell.
![]()
[July 18]
Solution:
Given: Édge length (a) ![]()
![]() , Atomic mass
, Atomic mass ![]()
![]() , Density
, Density ![]()
![]()
To find: ![]()
![]() Number of silver atoms in the unit cell
Number of silver atoms in the unit cell
Formula: ![]()
![]() Density
Density ![]()
![]()
Calculation: From formula,
![]()
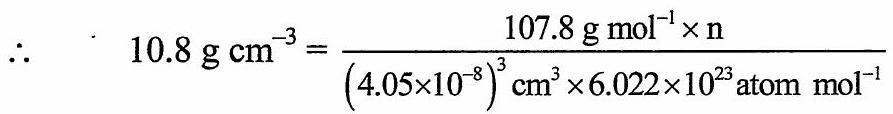
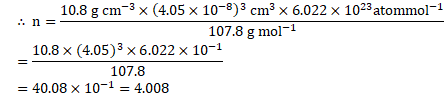
![]()
![]() Number of silver atoms in unit cell
Number of silver atoms in unit cell ![]()
![]()
Ans: The number of silver atoms in the unit cell is 4 .
- Unit cell of a metal has edge length of

 and density of
and density of 
 . Determine the type of crystal lattice. [Atomic mass of metal
. Determine the type of crystal lattice. [Atomic mass of metal 
 ]
]
[Mar 20]
Solution: Refer Subtopic 1.5: Numerical Q. No. 2.
- Gold crystallises into face-centred cubic cells. The edge length of unit cell is

 . Calculate the density of gold. [Molar mass of gold
. Calculate the density of gold. [Molar mass of gold 
 ]
]
Solution:
Given:
![]()
To find: ![]()
![]() Density
Density ![]()
![]()
Formula: ![]()
![]() Density
Density ![]()
![]()
Calculation: For an fcc lattice, number of atoms per unit cell is 4 .
![]()
From formula,
Density, ![]()
![]()
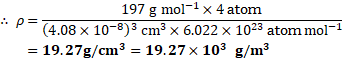
Ans: Density of gold is ![]()
![]() .
.
- Silver crystallizes in fce structure. If edge length of unit cell is

 , calculate density of silver (Atomic mass of
, calculate density of silver (Atomic mass of 
 ).
).
Solution:
Given:
Edge length (a) ![]()
![]()
To find:
Atomic mass of ![]()
![]()
Formula:
Density ![]()
![]()
Density ![]()
![]()
Std. XII Sci: Board Questions with Solutions
Calculation: . For an fcc lattice, number of atoms per unit cell is 4.

Ans: Density of silver is ![]()
![]() .
.
1.7. Packing efficiency
- Gold occurs as face centred cube and has a density of

 . Calculate atomic radius of gold. [Molar mass of
. Calculate atomic radius of gold. [Molar mass of 

Solution:
Given: ![]()
![]() Type of unit cell is fcc.
Type of unit cell is fcc.
Density of gold ![]()
![]()
Molar mass of gold ![]()
![]()
To find: ![]()
![]() Radius of gold atom
Radius of gold atom ![]()
![]()
Formulae: i. Density ![]()
![]() ii. For fcc unit cell,
ii. For fcc unit cell, ![]()
![]()
Calculation: For fcc unit cell, ![]()
![]() .
.
Using formula (i),
Density ![]()
![]()
![]()
![]()
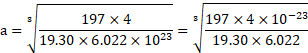
Using formula (ii),
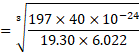
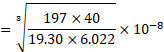
![]()
![]()
Ans: Radius of gold atom ( ![]()
![]() ) is
) is ![]()
![]() .
.
![]()
- Niobium crystallises as body centred cube (bcc) and has density of

 . Calculate the atomic radius of niobium.’
. Calculate the atomic radius of niobium.’
[Given: Atomic mass of niobium =93]
[Mar 15]
Solution:
Given: ![]()
![]() Type of unit cell is bcc.
Type of unit cell is bcc.
Density of niobium ![]()
![]()
Molar mass of niobium ![]()
![]()
To find: ![]()
![]() Radius of niobium atom (
Radius of niobium atom ( ![]()
![]() )
)
Formulae: i. Density ![]()
![]()
ii. For bcc unit cell, ![]()
![]()
Calculation: For bcc unit cell, ![]()
![]() .
.
Using formula (i),
Density ![]()
![]()
![]()
![]()
![]() .
.
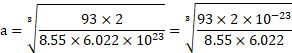
Using formula (ii), ![]()
![]()
![]()
![]()
![]()
![]()
Ans: Radius of niobium atom ( ![]()
![]() ) is
) is ![]()
![]() .
.
- Calculate the number of atoms and unit cells present in

 of Niobium if it forms body centred cubic structure. The density of Niobium is
of Niobium if it forms body centred cubic structure. The density of Niobium is 
 and edge length of unit cell is
and edge length of unit cell is 
 .
.
Solution:
[July 22]
Given:
Type of unit cell is bcc.
Density ( ![]()
![]() ) of niobium is
) of niobium is ![]()
![]() ,
,
Edge length ![]()
![]() ,
,
Mass of niobium ![]()
![]()
To find: i. Number of atoms in ![]()
![]() of niobium
of niobium
ii. Number of unit cells in ![]()
![]() of niobium
of niobium
Formulae: i. Number of atoms in ![]()
![]() of metal
of metal ![]()
![]()
ii. Number of unit cells in ![]()
![]() of metal
of metal ![]()
![]()
Calculation: i. ![]()
![]() For bcc unit cell,
For bcc unit cell, ![]()
![]() .
.
Using formula (i),
Number of atoms in ![]()
![]() of niobium
of niobium
![]()
ii. Using formula (ii),
Number of unit cells in ![]()
![]() of niobium
of niobium
![]()
Ans: i. Number of atoms in ![]()
![]() of niobium is
of niobium is ![]()
![]() .
.
ii. Number of unit cells in ![]()
![]() of niobium is
of niobium is ![]()
![]() .
.