MARCH 2019 PAPER – l
SCIENCE AND TECHNOLOGY PAPER-I
Note : (i) All questions are compulsory.
(ii) Draw scientifically, technically correct labelled diagram wherever necessary.
(iii) Start writing each main question on new page.
(iv) Figure to the right indicate full marks.
(v) For each MCQs (i.e. Q. No. 1-B) evaluation would be done for first attempt only.
(vi) For each MCQ correct answer must be written along with its alphabet.
Eg : (i) (a) …………………………
(ii) (b)……………………..
(iii) (d) ………………………….
Q. 1. (A) Answer the following questions:
(1) Write proper answer in the box:
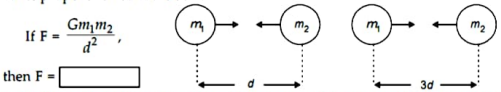
Ans.
If , then
(2) In Dobereiner’s triads , the atomic masses of lithium and potassium are 6.9 and 39.1 respectively, then what will be the atomic mass of sodium?
Ans.
Average atomic mass of sodium Atomic mass of
(3) State whether the given statement is true or false :
A concave lens is a converging lens.
Ans.
False. Concave lens is a diversing lens
(4) By considering first correlation complete the second correlation :
Hubble telescope : high from earth surface
Revolving orbit of Hubble telescope :
Ans.
Revolving orbit of hubble telescope
Revolving orbit of hubble telescope :
(5) Find the odd man out :
Tinning, Anodization, Alloying, Froth floatation.
Ans.
Froath Floatation.
(B) Choose the correct alternative :
(1) The reaction of iron nail with copper sulphate solution is reaction.
(a) combination
(b) decomposition
(c) displacement
(d) double displacement
Ans.
(c) Displacement reaction.
(2) Observe the following diagram and choose the correct alternative :
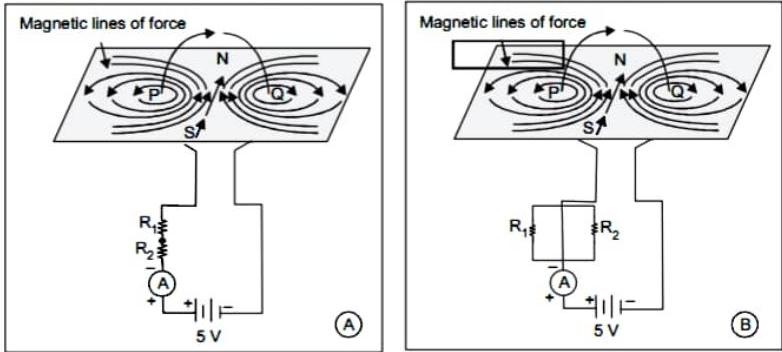
(a) The intensity of magnetic field in A is larger than in B.
(b) The intensity of magnetic field in is less than in .
(c) The intensity of magnetic field in A and B is same.
(d) The intensity of magnetic field in is less than in .
Ans.
(d) The intensity of magnetic field in is less than in .
(3) A ray of light makes an angle of with the surface of the glass slab. Its angle of incidence will be
(a)
(b)
(c)
(d)
Ans.
(b) .
(4) Water expands on reducing its temperature below. .
(a) 0
(b) 4
(c) 8
(d) 12
Ans.
(b) .
(5) The carbon compound is used in daily life is
(a) Edible oil
(b) Salt
(c) Carbon dioxide
(d) Baking soda
Ans.
(a) Edible oil.
Q. 2. Attempt any five of the following questions :
(1) Two tungsten bulbs of power and work on potential difference. If they are connected in parallel, how much current will flow in the main conductor?
Ans.
(2) Give scientific reason :
In the electric equipment producing heat e.g., iron, electric heater, boiler, toaster etc., and alloy such as nichrome is used, not pure metals.
Ans.
(3) A metal ball of mass falls from a height of . How much time it will take to reach the ground ?
Ans.
(4) Write names of first four homologous series of alcohols :
low resistivity. An alloy such as Nichrome has high resistivity and can be heated to a high temperature without undergoing oxidation. Thus, nichrome is preferred over other pure metals and are used for making coil for devices working on heating effect of electric current.
(3) Given, Mass .
Distance travelled by the ball
Initial velocity of the ball
Gravitational acceleration
Newton’s second equation of motion gives
The ball takes 10 seconds to reach the ground.
(4)
(5)
Points | Answers | ||||
(i) Position of the object | Between and | ||||
(ii) Position of the image |
| ||||
(iv) Nature of the image | Virtual and erect |
(6) Out of sodium and sulphur; sodium is a metal. So, sodium reacts with oxygen to form sodium oxide.
(7) (i) The description given in question is of hydraulic separation method.
(ii) Digram of hydraulic separation methods :
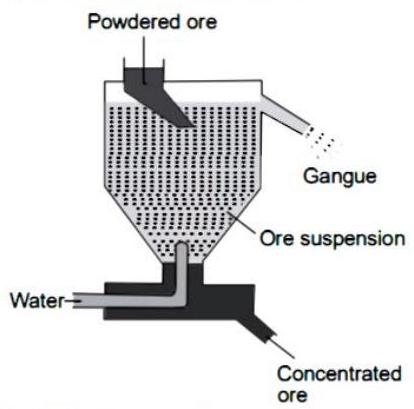
Q. 3. Attempt any five of the following questions :
(1) What would be the value of ‘ ‘ on the surface of the earth if its mass was twice and its radius half of what it is now ?
Ans.
(1) Given, Mass of the earth
Formula :
Thus,
The value of ‘ ‘ on the earth surface if its mass was twice and its radius was half of original value is, i.e., 8 times the original value of .
(2) Write merits of Mendeleev’s periodic table.
Ans.
Merits of Mendeleev’s periodic table are as follows :
(i) Atomic masses of some elements were revised to give them proper place in the periodic table in accordance with their properties. For example, the previously determined atomic masses of beryllium was changed to the correct value 9.4 and beryllium was placed before boron.
(ii) Mendeleev kept vacant places in the periodic table for elements not discovered till then. Three of these unknown elements were given the names eka-boron, ekaaluminium and eka-silicon from the known neighbours and their atomic masses were indicated as 44, 68 and 72 respectively. Their properties were also predicted. Later on, these elements were discovered and named as scandium (Sc), gallium and germanium respectively. The properties of these elements matched well with those predicted by Mendeleev.
(iii) There was no place reserved for noble gases in Mendeleev’s original periodic table. However when noble gases such as helium, neon and argon were discovered towards the end of nineteenth century, Mendeleev created the ‘zero’ group without disturbing the original periodic table in which the noble gases were fitted very well.
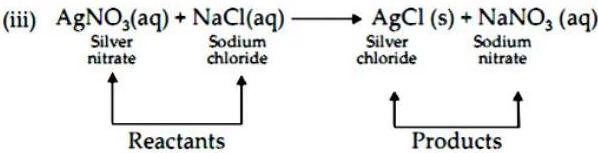
(3) Study the following chemical reaction and answer the questions given below :
(i) Identify and write the type of chemical reaction.
(ii) Write the definition of the above type of chemical reaction.
(iii) Write the names of reactants and products of the above reaction.
Ans.
(i) Double displacement reaction.
(ii) The reaction in which the ions in the reactants are exchanged to form a precipitate are called double displacement reactions.
(4) Explain the following temperature s. time graph:
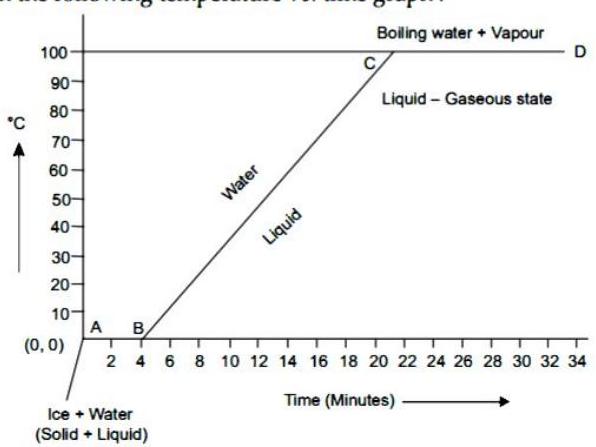
Ans.
(i) The graph shown is a temperature-time graph.
(ii) AB shows that at constant temperature of ice changes to water.
(iii) The temperature at which ice changes to water is called melting point of ice i.e. .
(iv) Now, water at starts heating and it’s temperature gradually increases to .
(v) shows increase in temperature from to without changes in state.
(vi) CD shows that, at constant temperature of water changes to vapour.
(vii) The temperature at which water changes in vapour is called boiling point of water i.e., .
(5) Surabhi from std. uses spectacle. The power of the lenses in her spectacle is . Answer the following questions from the given information :
(i) Identify the type of lenses used in her spectacle.
(ii) Identify the defect of vision Surabhi is suffering from.
(iii) Find the focal length of the lenses used in her spectacle.
Ans.
Given, power of lenses in spectacle used by Surabhi is ![]() .
.
(i) Convex lenses are used in Surabhi’s spectacles.
(ii) Far sightedness or hypermetropia.
(iii) We know, ![]() Power
Power ![]()
![]() Focal length
Focal length ![]()
(6) Complete the following table:
Sr. No. | Common Name | Structural Formula | IUPAC Name |
1. | Ethylene | ……………………… | |
2. | …………………….. | Ethanoic acid | |
3. | Methyl alcohol | ………………….. | Methanol |
Ans.
Sr. No. | Common Name | Structural Formula | IUPAC Name |
1. | Ethylene | Ethene | |
2. | Acetic acid | Ethanoic acid | |
3. | Methyl alcohol | Methanol |
(7) What is meant by space debris ? Why there is need to manage the debris ?
Ans.
Space debris : In addition to the artificial satellite, some other objects are also revolving around the earth like non-functional satellites, parts of the launcher detached during launching in earth’s orbit are called space debris. This debris can be harmful to the artificial satellites. It can collide with these artificial satellites or space crafts and damage them. Thus, space debris should be managed.
Q. 4. Answer any one of the following questions :
(1) Taking into consideration the period of the elements given below, answer the following questions :
Elements | Atomic Radius (pm) |
O | 66 |
B | 88 |
C | 77 |
N | 74 |
Be | 111 |
Li | 152 |
(i) Arrange the above elements in a decreasing order of their atomic radii.
Ans.
Elements | |||||||||||
Atomic Radius (pm) | 152 | 111 | 88 | 77 | 74 | 66 |
(ii) State the period to which the above elements belong.
Ans.
Elements O, B, C, N, Be, Li belong to 2nd period.
(iii) Why this arrangement of elements is similar to the above period of modern periodic table?
Ans.
Yes, this arrangement of elements is similar to the above period of modern periodic table. As we move from left to right within a period the atomic number increases one by one, meaning the positive charge on the nucleus increases by one unit at a time, but the electrons are added to the same orbit thereby increasing the pull towards the nucleus which decreases the size of the atom.
(iv) Which of the above elements have the biggest and the smallest atom ?
Ans.
Li has the biggest atom and has the smallest atom.
(v) What is the periodic trend observed in the variation of atomic radius while going from left to right within a period?
Ans.
Atomic radius decreases from left to right within a period due to increase in the effective nuclear charge.
(2) The observation made by Swarali while doing the experiment are given below. Based on these write answers to the questions :
Swarali found that the light ray travelling from the denser medium to rarer medium goes away from the normal. If the angle of incidence is raised by Swarali, the angle of refraction went on increasing. However, after certain value of the angle of incidence the light ray is seen to return back into the denser medium.
(i) What is the specific value of called?
Ans.
Critical Angle
(ii) What is the process of reflection of incident ray into denser medium called?
Ans.
Total internal reflection
(iii) Draw the diagrams of three observations made by Swarali.
Ans.
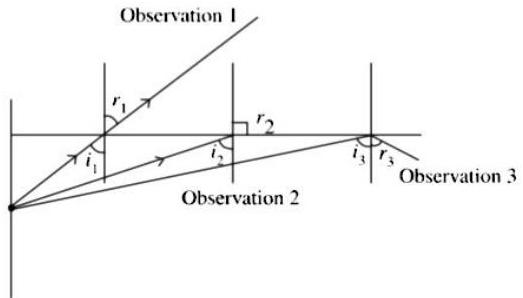
Observation 1 : When then refraction takes place and refracted ray moves away from normal i.e., .
Observation 2 : When then refracted ray becomes perpendicular to normal i.e., .
Observation 3 : When then total internal reflection takes place , where, is the critical angle.